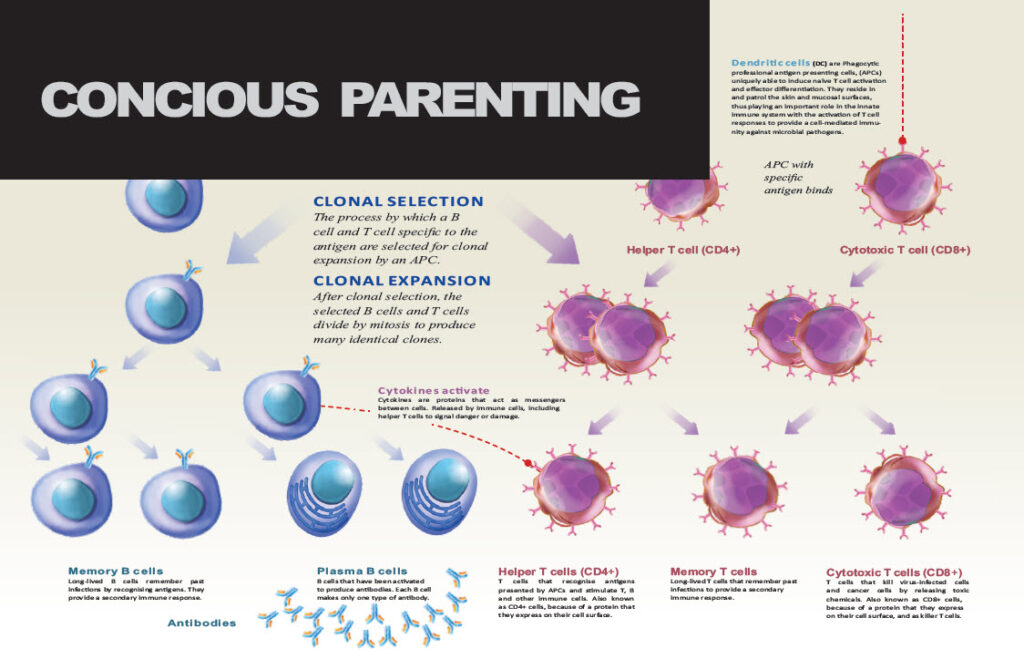
CD4+T Cells
Differentiation and Functions
CD4+T cells are crucial in achieving a regulated effective immune response to pathogens.
CD4+T cells carry out multiple functions, ranging from activation of the cells of the innate immune system, B-lymphocytes, cytotoxic T cells, as well as non-immune cells and also play critical role in the suppression of immune reaction.
The control of immune responses is important for proper functioning of the immune system and necessary to avoid immunopathology, largely manifesting as allergic and autoimmune diseases.
Interleukin 4 (IL-4) plays a pivotal role in shaping the nature of the immune responses. Upon activation, naïve peripheral CD4+ T cells begin to synthesise and secrete cytokines. These cytokines serve as autocrine growth and differentiation factors and, as a consequence, naïve T cells proliferate and differentiate into effector cells.
Based on the pattern of the cytokines that they secrete, distinct subsets of effector helper T (Th) cells can be distinguished. Type 1 Th cells (Th1) secrete IL-2, interferon gamma (IFN-y) and tumour necrosis factor (TNF), whereas Type 2 Th cells (Th2) produce IL-4, IL-5, IL-6 and IL-13. IL-4 is a 15-Kd polypeptide with pleiotropic effects on many cell types. It’s receptor is a heterodimer composed of an α (alpha) subunit, with IL-4 binding affinity, and the common y (gamma) subunit which is also part of other cytokine receptors. In T cells, binding of IL-4 to its receptor induces proliferation and differentiation into Th2 cells.
Immunity in the newborn is characterised by minimal Th1 function but an excess of Th2 activity. Since Th1 lymphocytes are important to counter microbes and Th2 cells favour allergies, the newborn faces susceptibility to microbial infections and allergic reactions.
Delayed maturation of certain dendritic cells leads to limited IL-12 production during the neonatal period. The Th2 cytokine locus of neonatal CD4+ T cells is epigenetically poised for rapid and robust production of IL-4 and IL-13. Together, these circumstances lead to efficient differentiation of Th2 cells and the expression of an IL-4Rα/IL-13Rα1 heteroreceptor on Th1 cells. Upon rechallenge, Th2 cells rapidly produce IL-4 which utilises the heteroreceptor to drive apoptosis (death) of Th1 cells causing the Th2 bias of neonatal immunity.
Landmark experiments by Sir Peter Medawar’s group in the 1950s demonstrated that neonatal exposure to antigen (Ag) (vaccination) leads to a lack of responsiveness to the same Ag during a later encounter [Billingham RE, et al. Actively acquired tolerance of foreign cells. Nature 1953;172:603–606. PubMed:13099277]
Th1
Following activation, naive CD4 T cells differentiate towards Th1 in the presence of interleukin 12 (IL-12), which upregulates interferon-gamma (IFNy) via Stat 4, leading to IFNy-mediated Stat1 activation and induction of the Th1 lineage determining transcription factor Tbet.
The main effector functions of Th1 cells are in cell-mediated immunity and inflammation, including the activation of cytolytic and other effector functions of other immune cells such as macrophages, B cells and CD8+ cytotoxic T lymphocytes (CTLs). Early studies by Mosmann et al. have demonstrated that CD4+ T cells can polarise into two different subsets, T helper 1 (Th1) and T helper 2 (Th2), based on the profile of the cytokines secreted. Th1 cells are mainly characterised by the production of large quantities of interferon-gamma (IFN y), while Th2 cells secrete interleukin (IL)-4, IL-5, and IL-13.
The death of Th1 cells is driven by IL-4 produced by the Th2 secondary cells.
Th1 cells are the main agents in delayed type hypersensitivity immune response through activation of macrophages and are crucial to host defence against intracellular pathogens. They mainly secrete IFNγ, lymphotoxin α (Lfα) and IL-2.
IFNγ is essential for the activation of mononuclear phagocytes, including macrophages, microglial cells, thereby resulting in enhanced phagocytic activity (H. W. Murray, B. Y. Rubin, and S. M. Carriero, Human mononuclear phagocyte antiprotozoal mechanisms: Oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii, Journal of Immunology, vol. 134, no. 3, pp. 1982–1988, 1985) and inducer of major histocompatibility complex class II molecule expression.
Th1 cells derive from the alpha:beta lineage of T cells and recognise antigens presented by major histocompatibility complex (MHC) class I or II molecules. Th1 cells play important roles in the identification and eradication of intracellular pathogens such as viruses and bacteria, including Mycobacterium tuberculosis, Mycobacterium leprae (leprosy)and Leishmania.
Th2
Th2 by contrast differentiates in response to IL-4, which activates Stat 6, resulting in induction of GATA3. Th2 cells mount immune response to extracellular parasites, including helminthes and play major role in induction and persistence of asthma as well as other allergic diseases.
The key effector-cytokines include IL-4, IL-5, IL-9, IL-13, IL-10, IL25 and amphiregulin. IL-4 is a major cytokine involved in allergic inflammation.
It is involved in IgE switching and secretion by B cells. IL-4 also upregulates low-affinity IgE receptor (FcεRI) on B-lymphocytes and mononuclear phagocytes and also high-affinity IgE receptor (FcεRII) on mast cells and basophils, with subsequent degranulation of the cells and release of several active metabolites, including histamine and serotonin.
Th2 responses are important to limit extracellular pathogens and can also counterbalance Th1 immune responses. Th2 cells are more efficient in mounting humoral immune responses, triggering the production of antibodies and promoting eosinophil infiltration.
Th0 cells can differentiate into Th1 or Th2 subsets at very early stages of cell activation. Since Th1 cells are fully capable of secreting cytokines, they can in turn inhibit Th2 cell differentiation and vice versa.
Overproduction of Interleukin 4 (IL-4) is associated with allergies.
DENDRITIC CELLS
Dendritic cells form an important part of the immune system. When something foreign enters your body the dendritic cells sample it (who, what are you?) and then place a piece of it on their outside surface (membrane). This is then presented to other cells in order to give the immune response instructions as to what to do. 99.99 per cent of the time our bodies don’t need to do anything – unless there is a threat from germs (pathogens / antigens.)
If so, the pathogen is presented to a naïve CD4 T-Cell (naïve meaning that it hasn’t differentiated and developed yet). The activation that occurs depends on the chemicals (cytokines) that it meets as well as the receptors. From here the immune system can proceed in several directions:
Th1: The naïve CD4 T-Cell can develop a Th1 profile in which case T cells will then play the leading role in the immune response. (CD4 T cells become Th1 cells because of signals they receive from interleukin 12 (IL-12.) This is an inflammatory process and is what protects us from pathogens. Basically, this is what we want to happen when we are attacked by a microbe.
Th1 cells protect us from bacteria; importantly from Mycobacterium tuberculosis, Mycobacterium leprae (leprosy) and Leishmania (parasites from sand-flies). The main functions of Th1 cells are in cell-mediated immunity and inflammation, including the activation and functions of other immune cells such as macrophages, B cells, and CD8+ cytotoxic T lymphocytes (CTLs).
Th2 : Then there is the Th2 profile which is designed to protect us from intracellular pathogens – from parasites. (This activates eosinophils which are responsible for many allergies.) When Th1 and Th2 work together a high level of inflammation is evident. Finally when the infection has been overcome the Th2 messengers (cytokines) tell the Th1 cells to shut down and that the job is done.
In the vitally important study 2010 by Dr Zaghouani PMID 19846341 / PMC2787701 new born mice were injected with an antigen. Because the dendritic cells were sufficiently immature the new born mice only made a small amount of IL-12. (Normally, as an adult, if you get injected with a vaccine you will make massive amounts of IL-12 and you will mount an effective immune response.) This is important because what he saw in these mice is what is seen in the outcome in humans. When the vaccine is done early – even with just an antigen and no adjuvant – in one of those windows of opportunity something interesting happens:
When they re-vaccinated the same mouse as an adult what they found was that it mounted a Th1 and a Th2 response – but because of the way that the Th1 cells had been programmed earlier the Th2 cells basically killed off the Th1 cells – and compromised the immune system’s ability to mount an effective immune response to bacteria. (Became tolerogenic instead of immunogenic.)
The abnormal response in the adult mice occurred because, as infant mice, they were vaccinated too early with injected antigen. In this case they had their immune systems skewed toward Th2 and could do nothing about it for the rest of their lives.
Likewise a human infant’s immune system is fully capable of responding – It can respond through Th1 and it can respond through Th2. If, however, it is stimulated too early (within the first three to six months) you get an underdeveloped – mutated receptor on the Th1 cell membrane. (An epigenetically hypo-methylated area on the surface of the Th1 cells.) When an infant has been vaccinated everything appears fine because it makes antibodies and nobody knows that there is a problem. This is really important, because all reactions don’t occur and become obvious right away. When re-exposed as adults to the same germs the immune response is however skewed and the Th2 cells then destroy the Th1 cells. (apoptosis). Autoimmune diseases are a direct consequence of a skewed immune response.
From a practical point of view, the diminished ability to produce Th1 responses makes the body more vulnerable to infections and Th2 cell dominance makes the adult vulnerable to allergic reactions.
Infants in South Africa are given Tuberculosis (BCG) Vaccine and Oral Polio Vaccine (OPV0) at birth.
6 weeks: Oral Polio Vaccine (OPV1), Rotavirus Vaccine (RV1) , Diptheria, Tetanus, Acellular Pertussus, Inactivated Polio Vaccine and Haemophilius Influenza Type B and Hepatitis B (DTaP-IPV – Hib1), (Hep B1,Pneumococcal Conjugated Vaccine (PCV 1)
10 weeks: DTaP-IPV – Hib – HBV(2) Diptheria, Tetanus, Acellular Pertussus, Inactivated Polio Vaccine and Haemophilius Influenza Type B and Hepatitis B combined.
14 weeks: Rotavirus Vaccine (RV2), Pneumococcal Conjugated Vaccine (PCV 2), DTaP-IPV – Hib – HBV(3) Diptheria, Tetanus, Acellular Pertussus, Inactivated Polio Vaccine and Haemophilius Influenza Type B and Hepatitis B combined.
6 months: Measles Vaccine.

The transcription factor T-bet (Tbx21) plays a major role in adaptive immunity and is required for optimal IFN-γ production by DCs.
Heterodimer: The key difference between homodimer and heterodimer is that homodimer is a protein made from two identical proteins, while heterodimer is a protein made from two different proteins.
GATA3: The GATA3 transcription factor is critical for the embryonic development of various tissues as well as for inflammatory and humoral immune responses and the proper
functioning of the endothelium of blood vessels. GATA3 plays a central role in allergy and immunity against worm infections.
INTERLEUKIN 4: IL-4 regulates eosinophil recruitment, inhibits macrophages, switches eiscosanoid production in macrophage and dendritic cells; is produced by mast cells, basophils, eisinophils and neutrophils. IL-4 is an STAT-6 signalling dependent 20-kd glycoprotein, which shifts the Th balance toward a Th2-mediated response. Other activities include down regulation of IL-12 production, the Th1 suppression response, and the suppression or blockage of the monocytederived cytokines.
STAT proteins: STAT proteins are activated by the Janus family (JAKs) tyrosine kinases in response to cytokine exposure.
STAT 4: is predominantly activated in response to IL-12, which drives T-helper cell differentiation towards the Th1 and Th17 pathways.
STAT 6: is activated by IL-4, and IL-13 with their receptors that both contain the α subunit of the IL-4 receptor (IL-4Rα). STAT6- mediated signalling pathway is required for the development of T-helper type 2 (Th2) cells and Th2 immune response.
IgE: Immunoglobulin E plays a critical role in the allergic inflammatory process in diseases.
IFNγ: Interferon gamma or type II interferon, is a cytokine that is primarily secreted by activated T cells and natural killer (NK) cells, and can promote macrophage activation, mediate antiviral and antibacterial immunity, enhance antigen presentation, orchestrate activation of the innate immune system, coordinate lymphocyte endothelium interaction, regulate Th1/Th2 balance, and control cellular proliferation and apoptosis.
Transcription factor: (TF) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The function of TFs is to regulate—turn on and off—genes in order to make sure that they are expressed in the desired cells at the right time and in the right amount throughout the life of the cell and the organism.
Hypo-methylation: DNA hypomethylation increases both the activity of Th2 cells and the production of IgE. DNA hypomethylation is generally associated with an elevated frequency of gene rearrangements and chromosomal translocations as a consequence of increased homologous recombination (HR). HR between repeated sequences leads to chromosome rearrangement, including deletions, duplications, and translocations of large DNA segments with disastrous consequences.

Ray Lacey is a CranioSacral Therapist (CST) and artist. In recent years he has devoted his attention to writing, illustrating and producing books. He graduated from Natal Technikon (DUT) in graphic art and from the Witwatersrand Technikon (JUT) in graphic design. He lectured at the same faculty in illustration and then worked as a freelance illustrator for many years.
In the late 1990s he developed an interest in the interpretation of children’s drawings. This led him to study remedial therapy for children with learning difficulties within the Waldorf School movement. In 2001 he undertook training in CST and qualified in 2002. Much of his work focuses on social upliftment projects and the adverse effects of vaccines, especially in children.
YouTube link:
https://www.youtube.com/watch?v=x-NDsABpJB4
Website: www.knovax.org